Mueller and Hinton developed Mueller Hinton Agar (MHA) in 1941 for the isolation of pathogenic Neisseria species. Nowadays, it is more commonly used for the routine susceptibility testing of non-fastidious microorganism by the Kirby-Bauer disk diffusion technique.
Five percent sheep blood and nicotinamide adenine dinucleotide may also be added when susceptibility testing is done on Streptococcus species. This type is also commonly used for susceptibility testing of Campylobacter.
Composition of MHA
| Ingredients | In Gram/Litre |
|---|---|
| Beef Extract | 2.00 gm |
| Acid Hydrolysate of Casein | 17.50 gm |
| Starch | 1.50 gm |
| Agar | 17.00 gm |
| Distilled Water | 1000 ml |
Final pH 7.3 ± 0.1 at 25ºC
Principle of MHA
Mueller Hinton Media contains Beef Extract, Acid Hydrolysate of Casein, Starch and Agar. Beef Extract and Acid Hydrolysate of Casein provide nitrogen, vitamins, carbon, amino acids, sulphur and other essential nutrients. Starch is added to absorb any toxic metabolites produced. Starch hydrolysis yields dextrose, which serves as a source of energy. Agar is the solidifying agent.
The use of a suitable medium for testing the susceptibility of microorganisms to sulfonamides and trimethoprim is essential. Antagonism to sulfonamide activity is demonstrated by para-aminobenzoic acid (PABA) and its analogs. Reduced activity of trimethoprim, resulting in smaller inhibition zones and innerzonal colonies, is demonstrated on unsuitable Mueller Hinton medium possessing high levels of thymidine. Both the PABA and thymine/thymidine content in Mueller Hinton Agar are reduced to a minimum, thus markedly reducing the inactivation of sulfonamides and trimethoprim when the media is used for testing the susceptibility of bacterial isolates to these antimicrobics.
Uses of MHA
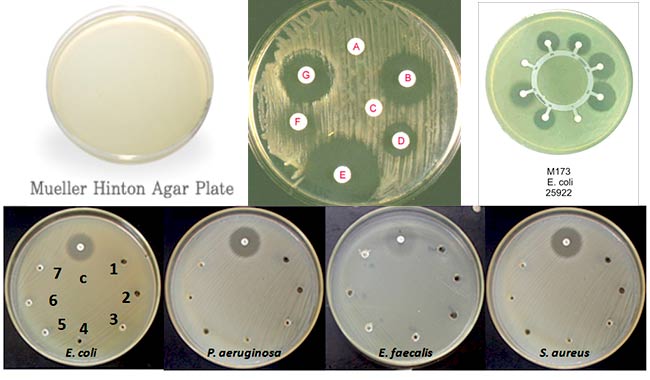
- The major use of Mueller Hinton Agar is for antimicrobial susceptibility testing. It has become the standard medium for the Bauer Kirby method and its performance is specified by the NCCLS.
- It can be used to cultivate Neisseria
- It is specified in FDA Bacteriological Analytical Manual for food testing, and procedures commonly performed on aerobic and facultative anaerobic bacteria.
Why MHA is used for antibiotic susceptibility testing?
- It is a non-selective, non-differential medium. This means that almost all organisms plated on here will grow.
- It contains starch. Starch is known to absorb toxins released from bacteria, so that they cannot interfere with the antibiotics. It also mediates the rate of diffusion of the antibiotics through the agar.
- It is a loose agar. This allows for better diffusion of the antibiotics than most other plates. A better diffusion leads to a truer zone of inhibition.
- MHA shows acceptable batch-to-batch reproducibility for susceptibility testing.
- MHA is low in sulfonamide, trimethoprim, and tetracycline inhibitors (i.e. concentration of inhibitors thymidine and thymine is low in MHA).
- Both the para-aminobenzoic acid (PABA) and thymine/thymidine content in Mueller Hinton Agar are reduced to a minimum, thus markedly reducing the inactivation of sulfonamides and trimethoprim when the media is used for testing the susceptibility of bacterial isolates to these antimicrobics.
Preparation of MHA
- Suspend 38 gm of the medium in one liter of distilled water.
- Heat with frequent agitation and boil for one minute to completely dissolve the medium.
- Autoclave at 121°C for 15 minutes. Cool to room temperature.
- Pour cooled Mueller Hinton Agar into sterile petri dishes on a level, horizontal surface to give uniform depth.
- Allow to cool to room temperature.
- Check for the final pH 7.3 ± 0.1 at 25ºC.
- Store the plates at 2-8 ºC.
Quality control of MHA
| Positive controls: | Expected results |
|---|---|
| Escherichia coli ATCC® 25922 | Good growth; pale straw colored colonies |
| Pseudomonas aeruginosa ATCC® 27853 | Good growth; straw colored colonies |
| Staphylococcus aureus ATCC® 25923 | Good growth; cream colored colonies |
| Negative control: | |
| Un-Inoculated medium | No change |
Limitations of MHA
- Numerous factors can affect results: inoculum size, rate of growth, medium formulation and pH. Strict adherence to protocol is required to ensure reliable results.
- Drug inactivation may result from the prolonged incubation times required by slow growers.
- Variation in the concentration of divalent cations, primarily calcium and magnesium affects result of aminoglycoside, tetracycline, and colistin test with P. aeruginosa isolates.

Is there a CLSI for QC of this agar media?
I need make 30 mullet Hinton plates how much powder and water would I need to make them ?
I think you will need about 300mL of prepared M-H agar. (10 mL per petri dish)
Add 10.4 g of powder to 300mL of distilled water, make sure all powder is dissolved before autoclaving @ 121 degrees Celsius for 15 minutes.
Good luck
you’ll need 11.4 g of powder ( 3.8 x 3 =11.4 g )
I would like to know whether an antibiotic in the form of powder can be used in agar for antimicrobial activity.
@ Buvana agar which is a solidifying agent in MHA will ultimately solidify the media hence you won’t have it in liquid form. Also, the powder shall diffuse or rather dissolve in liquid unless they’re immiscible.
I need to make 27 MHA plates. How much water and powder do I need? How much mL should I put in each petri dish? What is the orientation of the MHA plates if I store them in an incubator? Are they being stored/placed upside down or not? At what temperature should I put them in the incubator? Thank you!
What will happen if there will be excess amount of MHA agar?
@Feebie your questions is somehow opposite to that of @Preerika. They both revolve around the concentration of MHA in distilled water which should be 38g/L.
1. Less water or excess agar in MHA makes the media too solid to hard leading to cracking in the medium.
2. Excess water or less agar in MHA means less bacterial nutrients and a probability that MHA remain liquid increase with increase in water or decrease in agar.
that good but how starch absorb toxic from bacteria
Is is possible this medium not get solidify? If yes then what could be the reason?
sometimes, in summer MHA does not solidifies because of high temperature.
Agar is a solidifying agent, i will solidify. If the media is not solidified it will be considered as the MHA is expired. Any agar if it expires it will not solidify.
why only 9mm to 15mm antibiotic disc used for this antibiotics test
amazing work, is it possible to use mueller hinton broth for MIC determination ?
Dear sir,
I Went through your note, please let me know how much quantity of MH media pour in to each plate.
Hv Fruitful Day
Hi, usually agar plates require around 12ml of culture medium. Hope this is helpful!
how many plates will this be?
Volume depends on plate diameter
hlow sir, i just want to know how starch works in MH MEDIUM to absorb toxins and it gives protection to what?