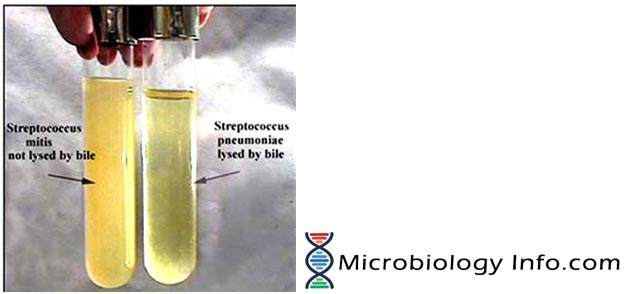
Bile Solubility Test is the test which differentiate Streptococcus pneumoniae (positive- soluble) from alpha-hemolytic streptococci (negative- insoluble). Streptococcus pneumoniae is bile soluble whereas all other alpha-hemolytic streptococci are bile resistant.
Principle of Bile Solubility Test
S. pneumoniae has an autolytic enzyme which can be demonstrated by allowing a broth culture to age in the incubator; at 24 hours the broth is turbid; after a few days the medium will become clear.
Bile or a solution of a bile salt (e.g., sodium desoxycholate) rapidly lyses pneumococcal colonies. Lysis depends on the presence of an intracellular autolytic enzyme, amidase. Bile salts lower the surface tension between the bacterial cell membrane and the medium, thus accelerating the organism’s natural autolytic process. Bile salts activate the autolytic enzyme which induces clearing of the culture.
Reagents for Bile Solubility Test
2% sodium deoxycholate (bile salt) solution
- Dissolve 2 gram of sodium deoxycholate into 100 ml sterile distilled water.
10% sodium deoxycholate (bile salt) solution
- Dissolve 10 gram of sodium deoxycholate into 100 ml sterile distilled water.
Procedure of Bile Solubility Test
Tube Method
- Prepare a heavy suspension of a pure culture in 2 ml of 0.85% saline.
- Divide the organism suspension into two tubes.
- Adjust the turbidity to that of 0.5-1 McFarland standard.
- To one tube (test tube), add 2 drops of 2% sodium desoxycholate and mix.
- To the other tube (control tube), add 2 drops of sterile water distilled water and mix.
- Leave both tubes for 10-15 minutes at 35-37°C.
- Observe for a clearing of turbidity in the tube containing 2% sodium deoxycholate.
- If negative, continue to incubate up to 3 hours. Observe again for clearing.
Plate Method
- Incubate the sample on 5% sheep blood agar for 12 to 24 hours.
- Place one to two drops of 10% sodium desoxycholate to the side of a freshly isolated colony (18 -24 hours) on 5% sheep blood agar.
- Gently wash the solution over the colony with dislodging the colony from the medium.
- Incubate the culture plate at 35-37°C for 30 minutes.
- Examine for lysis of colony (Disappearance of the colony).
Modified Bile Solubility Test (Rapid Test)
The rapid identification of microorganisms recovered in blood can provide information about the source of the infectious process and guide the specific therapeutic management of the septic patient.
- Blood (10%, vol/vol) was inoculated into one bottle each of tryptic soy broth and Thiol broth under vacuum with C02 and 0.025% sodium polyanetholsulfonate.
- On one-half of the slide, 1 drop of blood was mixed with 1 drop of 2% sodium deoxycholate.
- On the other half of the slide, 1 drop of blood was mixed with 1 drop of water, which served as the control.
- The slide was air-dried at ambient temperature.
- Gram staining was done and examined for cocci.
- The numbers of organisms present in the blood treated with sodium deoxycholate and in the untreated blood were compared.
Result Interpretation of Bile Solubility Test
Tube Method
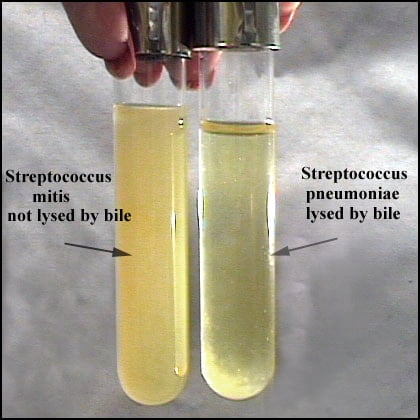
Positive Result: Suspension clears in tube labelled test and remains turbid in control tube.
Negative Result: Suspension remains turbid.
Note: Partial clearing (partial solubility) is not considered positive for S. pneumoniae identification.
Plate Method
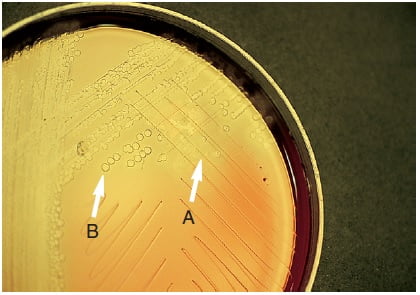
Figure: Bile solubility (desoxycholate) test. A, Colony lysed (Positive). B, Intact colony (Negative).
Positive Result: Colony disintegration or flattening of the colony within 30 minutes, leaving an alpha-hemolytic where colony may be located.
Negative Result: No change i.e. colonies remain intact.
Modified Bile Solubility Test
Positive Result: No cocci are seen in the test smear and control smear shows intact bacteria.
Negative Result: Cocci are seen in both test smear and control smear.
Quality Control for Bile Solubility Test
Positive Control: Streptococcus pneumoniae NCTC 12977
Negative Control: Streptococcus mitis NCTC 10712
Limitations of Bile Solubility Test
- Bile salts will not induce clearing of a killed culture or one that is too acid. Therefore saline suspensions of young cultures are used.
- The test should not be performed on old cultures, as the active enzyme may be lost.
- Normal autolysis of S. pneumoniae may be inhibited by a high concentration of bile salts being used. Evaporation may cause the reagent to become more concentrated, therefore affecting the test.
- When performing the bile solubility tube test using saline or unbuffered broth, it is essential to adjust the pH to neutral before adding the reagent in order to avoid false negative reactions.
- When testing using the plate method, care must be taken not to dislodge the colony being tested, therefore leading to false positive results.
References
- Bile solubility test. Right Diagnosis.
- Bile solubility. The University of Windsor.
- Bile Solubility Test. Bailey & Scott’s Diagnostic Microbiology, 13e. pp. 199.
- Monica Cheesbrough. District Laboratory Practice in Tropical Countries, Part 2, 2nd Edition. Pp. 63-64.
- Patrick R. Murray. Manual of Clinical Microbiology. 8th Volume 1. pp. 411-412.
- Patrick R. Murray. Modification of the Bile Solubility Test for Rapid Identification of Streptococcus pneumoniae. Journal of Clinical Microbiology, Feb. 1979, pp. 290-291.
- Bile Solubility Test. UK Standards for Microbiology Investigations.
- Bile Solubility Reagents. Remel.
- Bile Solubility Reagent. Dalynn Biologicals. Catalogue No. RB60.