Benedict’s Test is used to test for simple carbohydrates. The Benedict’s test identifies reducing sugars (monosaccharide’s and some disaccharides), which have free ketone or aldehyde functional groups. Benedict’s solution can be used to test for the presence of glucose in urine.
Some sugars such as glucose are called reducing sugars because they are capable of transferring hydrogens (electrons) to other compounds, a process called reduction. When reducing sugars are mixed with Benedicts reagent and heated, a reduction reaction causes the Benedicts reagent to change color. The color varies from green to dark red (brick) or rusty-brown, depending on the amount of and type of sugar.
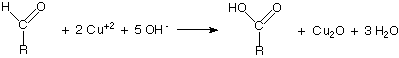
Benedict’s quantitative reagent contains potassium thiocyanate and is used to determine how much reducing sugar is present. This solution forms a copper thiocyanate precipitate which is white and can be used in a titration. The titration should be repeated with 1% glucose solution instead of the sample for calibration
Principle of Benedict’s Test
When Benedict’s solution and simple carbohydrates are heated, the solution changes to orange red/ brick red. This reaction is caused by the reducing property of simple carbohydrates. The copper (II) ions in the Benedict’s solution are reduced to Copper (I) ions, which causes the color change.
The red copper(I) oxide formed is insoluble in water and is precipitated out of solution. This accounts for the precipitate formed. As the concentration of reducing sugar increases, the nearer the final color is to brick-red and the greater the precipitate formed. Sometimes a brick red solid, copper oxide, precipitates out of the solution and collects at the bottom of the test tube.
Sodium carbonate provides the alkaline conditions which are required for the redox reaction. Sodium citrate complexes with the copper (II) ions so that they do not deteriorate to copper(I) ions during storage.
Complex carbohydrates such as starches DO NOT react positive with the Benedict’s test unless they are broken down through heating or digestion (try chewing crackers and then doing the test). Table sugar (disaccharide) is a non-reducing sugar and does also not react with the iodine or with the Benedict Reagent. Sugar needs to be decomposed into its components glucose and fructose then the glucose test would be positive but the starch test would still be negative.
Composition and Preparation of Benedict’s Solution
Benedict’s solution is a deep-blue alkaline solution used to test for the presence of the aldehyde functional group, – CHO.
Anhydrous sodium carbonate = 100 gm
Sodium citrate – 173 gm
Copper(II) sulfate pentahydrate = 17.3 gm
One litre of Benedict’s solution can be prepared from 100 g of anhydrous sodium carbonate, 173 g of sodium citrate and 17.3 g of copper(II) sulfate pentahydrate.
Procedure of Benedict’s Test
- Approximately 1 ml of sample is placed into a clean test tube.
- 2 ml (10 drops) of Benedict’s reagent (CuSO4) is placed in the test tube.
- The solution is then heated in a boiling water bath for 3-5 minutes.
- Observe for color change in the solution of test tubes or precipitate formation.
Result Interpretation of Benedict’s Test
If the color upon boiling is changed into green, then there would be 0.1 to 0.5 percent sugar in solution.
If it changes color to yellow, then 0.5 to 1 percent sugar is present.
If it changes to orange, then it means that 1 to 1.5 percent sugar is present.
If color changes to red,then 1.5 to 2.0 percent sugar is present.
And if color changes to brick red,it means that more than 2 percent sugar is present in solution.
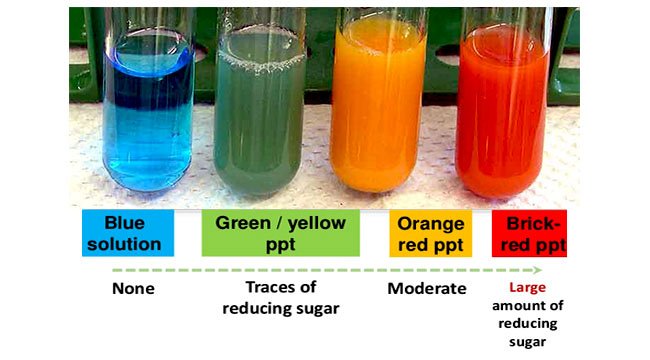
Positive Benedict’s Test: Formation of a reddish precipitate within three minutes. Reducing sugars present. Example: Glucose
Negative Benedict’s Test: No color change (Remains Blue). Reducing sugars absent. Example: Sucrose.
References
- National Institutes of Health, Testing for Lipids, Proteins and Carbohydrates- Benedict’s solution.
- Fayetteville State University- Biological Molecules: Carbohydrates, Lipids, Proteins.
- Harper College- Benedict’s Test.
- National Biochemicals Corp.- BENEDICT’S SOLUTION (MB4755).
- Science Olympiad- Use of Benedict’s Solution.
- Brilliant Biology Student 2015- Food Tests- Benedict’s Test for Reducing Sugars.
- BBC Bitesize- Chemistry- Carbohydrates.
- University of Manitoba- The Molecules of Life: Biochemistry- Carbohydrates.
- Northern Kentucky University- Benedict’s Reagent: A Test for Reducing Sugars.
- KNUST Open Educational Resources, Benedict’s Test – Qualitative Test in Carbohydrates.
- Mark Rothery’s Biology Web Site- Biochemical Tests.
- All Medical Stuff- Benedict’s test for reducing sugar.
- Hendrix College- Benedicts Test for Glucose.
- Info Please- Benedict’s solution.
- Mystrica- Benedict’s Test.
- Amrita Virtual Lab Collaborative Platform- Qualitative Analysis of Carbohydrates.
- Wikipedia.
Similar Posts:
- Oxidase Test- Principle, Uses, Procedure, Types, Result Interpretation, Examples and Limitations
- Indole Test- Principle, Reagents, Procedure, Result Interpretation and Limitations
- The Triple Sugar Iron (TSI) Test – Principle, Procedure, Uses and Interpretation
- Bile Solubility Test- Principle, Reagents, Procedure and Result Interpretation

What can I use to remove the orange copper stains left over in the test tube? Simple scrubbing with soap is hard to get rid of it. Thanks!
it is possible to get the same results if sucrose was used instead of glucose?
No, because sucrose is a table sugar and as this article states, table sugar is not reductive.
How do lipids react with ethanol
I want to test how freezing and thawing of food can affect its carbohydrate content. when pasta is frozen and thawed the starch turns into resistant starches so will the benedicts test be able to detect that?
the benedict test is not fantastic at testing starches in general due to their complexity. resistant starches, even less so. i’d recommend using an iodine test instead. the iodine test is designed to detect complex carbohydrates so it would detect your starches much better than benedict’s solution would.
I performed this particular experiment according to the outlined steps. The result l had was a dark brown color. What might be the cause of this result?
High concentration of reducing sugar
Which will be the colour of protiens if we add benedict’s solutoin 2 to 3 drops and caustic soda
If you add Benedict’s solution to caustic soda (sodium hydroxide), which is a strong base, without any reducing sugars present, you are likely to observe little to no color change. Benedict’s solution requires the presence of reducing sugars to undergo a chemical reaction that leads to the formation of a colored precipitate.
What is the differences between Benedict solution and Fehling’s solution
What are the precautions to take during the experiment
Use clean test tubes
Fehling’s reagent contains sodium potassium tartrate (Rochelle’s salt) in place of sodium citrate.
Hmm….Benedict’s Solution consists of copper sulfate, sodium citrate, and sodium carbonate. The copper ions in Benedict’s solution are reduced by the reducing sugars present in the test sample, leading to the formation of a colored precipitate.
Fehling’s Solution comes in two separate solutions, Fehling’s A and Fehling’s B. Fehling’s A contains copper(II) sulfate, while Fehling’s B contains sodium potassium tartrate and sodium hydroxide. The two solutions are mixed in equal proportions before use.
What is the different between Benedict’ and barfoe’d test
1 ml is approximately 20 drops. So 2 ml would be 40 drops. 10 drops would be 0.5 (1/2) ml. What should the protocol say?
There is one major problem in this writing….you’ve said Hydrogens are electrons. Hydrogens are protons…often, having given away an electron, they acquire a positive charge.
Id say this… reduction as we know it can also be the gain of hydrogen since it reduces non metals which would otherwise not lose electrons.
N₂+2H₂->2NH₃
We can heat directly so what’s effect on solution
Will it detect the presence of lactobionic acid?
What happens if you keep on heating the solution in boiling water bath for more than 5 minutes?
I made this mistake while working with Benedict’s Reagent, it burns the reagent and the substance you are testing creating an odd off colour that should not be used as sound results in a report. It is also quite difficult to clean any glassware after that mistake.
Do it is also known as fehling’s test for reducing sugars????
Hello Akash, the Benedict’s test is much more sensitive than the Fehling’s Test but they’re both tests for reducing sugars. 🙂
– fellow pre-med student trying to pass pharmacy
Does Formica acid give benedict test??
ohh no worries at all, hope you are enjoying your results XDXDXD
Hydrogens are not electrons, they are protons and often have a positive charge. Is it possible that the sugars are reducing sugars because they accept hydrogens instead of give them up?
I would like to know the precautions while using the solution.